Site Under Development, Content Population and SEO, Soft Launch 1st January 2020
The pH of the human body lies in a tight range between 7.35-7.45, and any minor alterations from this range can have severe implications.
Although the pH of blood ranges from 7.35-7.45, the pH of other body fluids is different. pH indicates the level of H+ ions, where low pH indicates too many H+ ions and high pH indicates too many OH- ions. If the pH levels drop below 6.9, it can lead to coma. However, different body fluids have different pH values. The pH of saliva is ranges from 6.5 to 7.5. After swallowing, the food reaches the stomach where upper and lower parts of stomach have different pH values. The upper part has a pH of 4−6.5, while the lower part is highly acidic with a pH of 1.5−4.0. It then enters the intestine which is slightly alkaline, with a pH of 7−8.5. Maintaining the pH values of different regions is critical for their function.
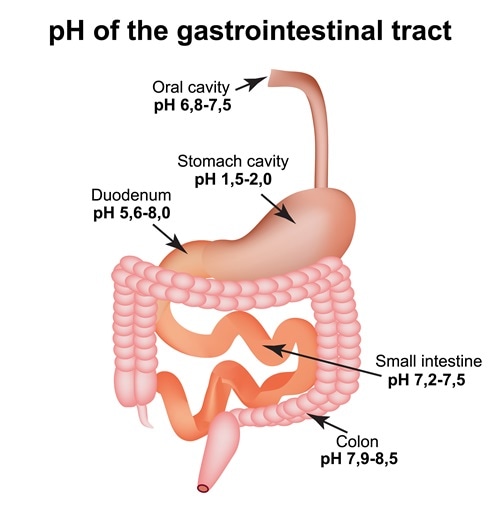
Different organs function at their optimal level of pH. For example, the enzyme pepsin requires low pH to act and break down food, while the enzymes in intestine require high pH or alkaline environment to function. Similarly, any increase or decrease in the blood pH can lead to several disorders.
pH is maintained in the body using primarily three mechanisms: buffer systems, respiratory control, and renal control.
Proteins form a part of the buffer system to regulate the pH levels. These proteins can act as H+ acceptors or donors because of the presence of basic or acidic groups. Similarly phosphate buffers also help in moderating the levels of pH. Buffers may help in regulating pH during minor physiological changes, such as during breath holding (which increases the CO2 in the blood), exercise (which increases lactic acid in the blood), or when gastric acid is secreted.
The pH of blood during normal conditions is 7.4. However, CO2 dissociates into carbonic acid in the tissues. Thus, presence of more CO2 makes the blood more acidic. That is the reason when we hold the breath for long durations, the CO2 levels increase in the blood lowering our pH leading to fainting. On the other hand during alkalosis or increased pH, the breathing may get slow in order to increase the CO2 levels and reduce the alkalinity. However, low breathing rate could also lead to low oxygen levels which could be detrimental. Thus, respiration provides an important control to regulate the pH levels.
The renal system regulates the pH of extracellular fluid. The changes in pH induced by the respiratory system are in minutes, while the changes induced by the renal system are in the order of days. If the acidity of the fluids is high, kidney secretes H+ ions, while if the carbonate ion levels are high it retains H+ ions and secretes HCO3 ions. Although this process is slow but it can prove an effective mode to regulate pH. One limitation of renal regulation is that the pH of urine cannot be below 4.4. Thus, strong acids can be removed by reacting with basic salts of phosphoric acid or by addition of base (NH3) to urine.
The abnormalities in acid-base balance are of two types: acidosis and alkalosis. In acidosis, the blood pH is low or there is too much acid in the blood, while in alkalosis, the blood pH is high or there is too much base in the blood. Acidosis and alkalosis may be caused either due to imbalance of acid-base secretion by the kidneys or altered levels of CO2 in the blood due to breathing disorders.