Site Under Development, Content Population and SEO, Soft Launch 1st January 2020
An epidermal nevus is a benign overgrowth of epidermal cells usually seen at birth or early in childhood. There is usually one or more nevi of varying diameter.
Epidermal nevi are classified by the type of cell involved. These include:
While keratinocytic nevi are called non-organoid nevi, and are the most common type, the other types of nevi comprise the non-organoid epidermal nevi.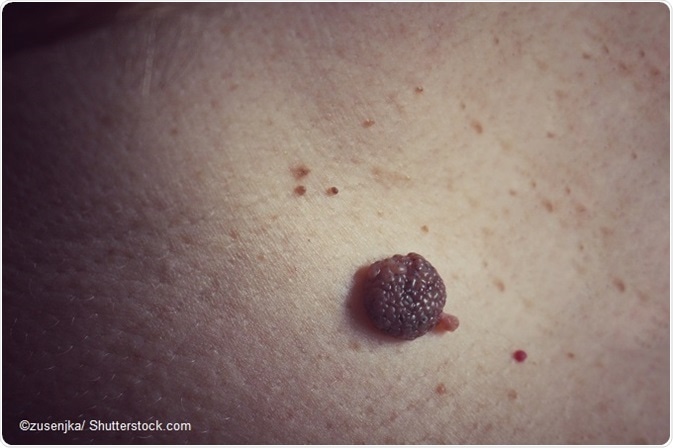
Most of the epidermal nevi and associated epidermal nevus syndromes are associated with various mutations in the abnormal cells which proliferate. About 40 percent of the keratinocytic epidermal nevi show cells with mutations in the FGFR3, PIK3CA, and HRAS genes.
The FGFR3 gene mutations alone account for 30 percent of all these lesions. This gene is the blueprint for the fibroblast growth factor receptor 3, a protein which is the interaction point for extracellular growth factors that regulate cell growth and differentiation. The mutated gene removes the need for attachment of the growth factor, which means the switch becomes autonomous. Constant growth and cell division therefore occur in these cells, resulting in overgrowth. Moreover, the programmed cell death process called apoptosis no longer operates to regulate the growth of the tissue. This gives rise to increased proliferation of the cells, leading to the development of an epidermal nevus.
Individuals affected by nevus sebaceous are mostly found to have HRAS gene mutations as well. Genes less commonly affected include the KRAS and NRAS genes, which bear relation to the HRAS gene.
The importance of the RAS and other genes involved in the genesis of epidermal nevi is that they bear the genetic imprint for the proteins that take part in cellular signals. This relaying of information about the environmental and intracellular milieu is crucial for cell regulation of all kinds of activity, including growth processes and cell division. In order for this process of signal transmission to take place properly, these protein messengers need to be activated. This turning on of the cellular switch in turn leads to a barrage of programmed reactions to achieve the desired cellular functions at the right time. These proteins are hotspot activators, in short.
The mutations in these essential genes result in the emergence of proteins which no longer require the external activator. This results in lack of control over cell division processes, leading to the overgrowth of skin cells, culminating in the development of epidermal nevi. The abnormal cells are embryonal ectodermal in origin, and therefore have the potential to give rise to lesions within the epidermis of the skin and in neural tissue, which is also an ectodermal derivative.
Research shows that these mutations are to be observed only within the cells of the nevus but not in the normal skin around. This is termed a mosaic, because of the partial presence of the altered gene within the cells of the same tissue in the same organism. This shows that the same individual has more than one genetic line in his cells, of which only one line produces the abnormal lesions. It is also a somatic mutation because it is acquired following conception. This means the parent does not transmit the genetic information via the germ line. It is also not inherited from the parental germ cells.
In some cases, the somatic mutation in the FGFR3 gene is located within a germ cell of one individual, in which case it becomes heritable. Affected children will show the presence of the mutation within every cell rather than only the cells of the epidermal nevus. This systematization leads to widespread bone anomalies of the ectoderm-derived tissues, in this case the bones.
Whether the abnormal cells lead to the development of an epidermal nevus or an epidermal nevus syndrome, with associated defects of the skeletal, ocular, and central nervous systems, depends on the time at which the post-zygotic mutations occur. This will also determine the lesion size, and whether tumors may arise from the epidermal nevus later. Since the ectodermal cell in embryonic life was destined to migrate to the surface and form the epidermis, following the lines of Blaschko, the mutation produces lesions of that area of the epidermis in the same direction.
Another type of epidermal nevus called the epidermolytic hyperkeratotic type arises from cells with mutated K1 and K10 (keratin) genes, a mosaic disorder of the suprabasal keratin layer. It is noteworthy that the offspring of these parents have a rare skin disorder which is generalized, and which may be the disseminated form of the mosaic disorder in the parents. Another mutation in the ATP2A2 gene which is responsible for the Darier disease has been found in the abnormal cells of an individual affected by acantholytic epidermal nevus, which has led to the hypothesis that the latter is a mosaic form of the former condition. Many mutations responsible for epidermal nevi remain undiscovered.