Site Under Development, Content Population and SEO, Soft Launch 1st January 2020
Adoptive T cell therapy is a type of immunotherapy with the ability to target cancer cells through inherent mechanisms of the immune system. The method works by infusing tumor-specific cytotoxic T cells into patients which will recognize, target and attack tumor cells.
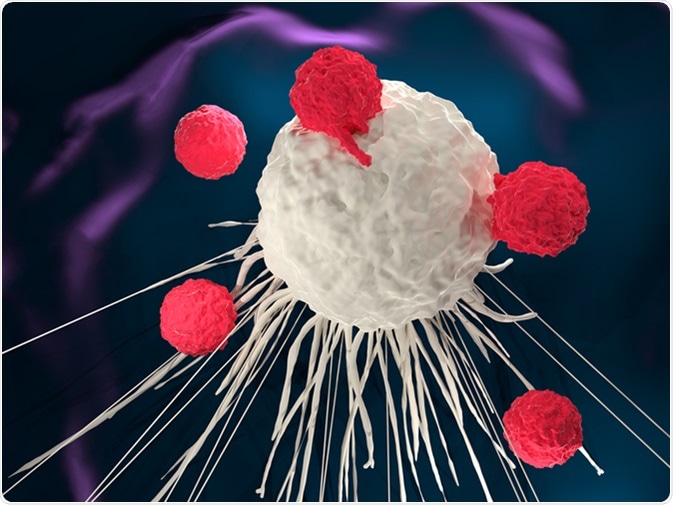
Adoptive T cell therapy methodologies include:
Adoptive T cell therapy ranges from the broad T-cell recognition capacities of tumor infiltrating lymphocytes to specific antigen response through engineered CAR T cells. The active immunization produced through developing cancer vaccines is also being trialed via adoptive T cell therapy.
The adoptive T cell therapy methodology with the most clinical history and demonstrated clinical response rates is the adoptive transfer of autologous tumor-infiltrating lymphocytes (TIL). TILs are extracted from tumors and cultured with interleukins, a type of cytokine messenger molecule, to form therapeutic TILs.
The tumor-infiltrating lymphocytes are then infused back into the patient where they re-infiltrate the tumor and cause tumor regression through the lysis of tumor cells. The earliest studies of infused TILs with interleukin‐2 were found to be 50-100 times more effective in destroying tumor cells than lymphokine‐activated killer (LAK) cells.
The treatment involves patient-specificity because TIL cultured from human tumors are able to lyse autologous cells from the same individual, but not allogeneic cells. Utilizing TILs for adoptive T cell therapy has the advantage of providing broad T-cell recognition for many tumor antigen types rather than the single specificity capacity of other techniques.
Current studies are attempting to minimize the time taken to culture TILs, improve the effector characteristics of T cells and identify biomarkers that allow for the selection of the most suitable patients that will respond well to this type of immunotherapy.
Chimeric antigen receptors (CAR) are engineered to redirect T cells to recognize and attack cancer cell targets. This type of therapy involves collecting T cells from the blood of the patient or donor and then genetically engineering the T cells to produce the surface receptors that allow the T cell to recognize and bind to specific cancer cell antigens. Viruses are often used as vectors for delivering the genes required for engineering the CAR T cells.
The CAR T cells are then cultured within the laboratory before being infused into the patient. The further multiplication of CAR T cells can occur within the body to produce an effective immunological treatment for hematologic malignancies.
Current studies are adapting the methodology to improve success rates in solid tumors by combining CAR T cells with other effector molecules.
Another method of adoptive T cell therapy is to remove T cells from the blood of patients that have received a cancer vaccine. The advantage of this technique is that by supplying active immunization in rare tumor antigen specific T cells, an effective therapy can be produced by expanding the number of primed T cells in the laboratory before infusion. One approach is to utilize tumor specific CD4+ Th1 cells for adoptive T cell therapy.
Th cells have wide functionality including the activation of antigen-specific effector cells and immune system cells, such as microphages, for antigen presentation.
By priming with antigens, Th cells are able to directly activate tumor antigen-specific cytotoxic T cells. The activation of antigen presenting cells helps to broaden immunity to other antigens within the tumor with the resulting epitope spreading connected to an increased survival benefit post immunotherapy in trials for melanoma and breast cancer treatment.