Site Under Development, Content Population and SEO, Soft Launch 1st January 2020
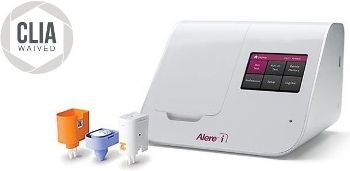
The Alere™ i Influenza A & B test revolutionizes rapid flu testing by providing highly accurate, molecular results in less than 15 minutes on the user-friendly Alere™ i platform. A rapid, molecular flu result with Alere™ i Influenza A & B empowers you to transform patient care in any setting.
Alere™ i Influenza A & B vs. PCR
PPA: Positive Percent Agreement
NPA: Negative Percent Agreement
Alere™ i Influenza A & B Performance vs. Culture
a. Flu A nucleic acid was detected in 58/66 False Positive specimens using an FDA-cleared molecular test.
b. Flu A nucleic acid was not detected in 1/2 False Negative specimens using an FDA-cleared molecular test.
c. Flu B nucleic acid was detected in 15/17 False Positive specimens using an FDA-cleared molecular test.
d. Flu B nucleic acid was not detected in 4/6 False Negative specimens using an FDA-cleared molecular test.
A new level of confidence in test sensitivity²,³
Faster than other molecular methods
The Alere™ i Influenza A & B kit contains all components required to carry out an assay for influenza A and B on the Alere™ i instrument. It tests for the qualitative detection and differentiation of influenza A and influenza B from nasal swab samples and viral transport media.
For more detail: https://www.news-medical.net/ads/abmc.aspx?b=1918